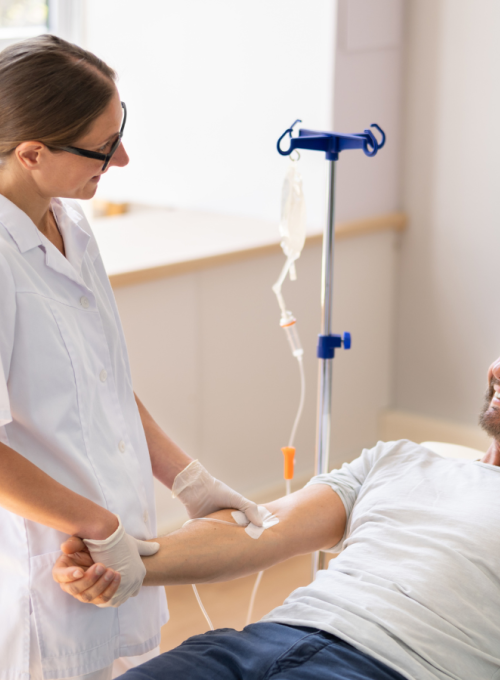
IV Drug (AV-1) Evaluation with Dengue Challenge Study (CIR362)
Outpatient study enrolling healthy adults 18-55 years old.
Johns Hopkins CIR is seeking volunteers for an outpatient study investigating a new intravenous (IV) drug called AV-1. This drug is being tested to see if it will treat or prevent dengue infection. Dengue virus is spread by mosquitoes and affects millions of people globally. While many dengue infections are asymptomatic or produce only mild symptoms like high fever, headache, body aches, nausea, and rash, the virus can also cause more severe illness, and even death. The number of dengue cases has increased in recent years, with half of the world's population being at risk of infection.
Study breakdown:
- 5 months long
- at least 2 screening visits to determine eligibility
- one IV infusion visit where participants receive study drug or placebo (IV infusion takes one hour, study visit will take 6-8 hours)
- one dengue virus challenge visit
- 15 follow-up appointments
Who can participate?
- Healthy adults aged 18-55
- BMI between 18.5 and 34.9 (BMI calculator)
- Must be willing to use certain birth control methods during the study.
- Must be available to visit the CIR for scheduled appointments.
- NOT pregnant, NOT breastfeeding, NOT planning travel to Dengue/Zika zones during the study.
- Other study criteria will be reviewed at in-person screening visits
If enrolled, you will receive:
- Up to $3425 for your time and participation.
- Parking stickers or MTA tokens for in-person visits.
Screening Dates
Contact us for new screening dates!
Screening Location(s)
CIR Outpatient Clinic @ Johns Hopkins East Baltimore Campus
855 N. Wolfe Street Suite 601
Baltimore, MD 21205
Study Period
5 months
Compensation
up to $3425 for completion of all study procedures
