Yellow Fever Outpatient Study (CIR 377)
The Johns Hopkins CIR is looking for healthy volunteers to participate in A Phase 3 randomized, modified double-blind, active-controlled study to assess the safety of a new yellow fever vaccine compared to the currently licensed one, in adults aged 18 years up to 60 years.
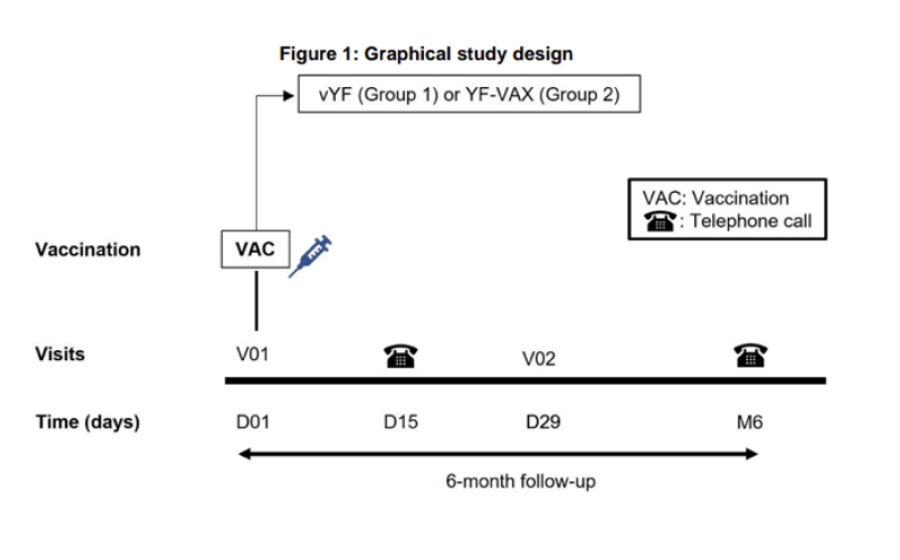
Eligible, consenting adults will receive a single dose of the investigational vaccine (vYF) or the currently licensed vaccine YF-VAX) in a 3:1 ratio.
The duration of the trial is 6 months There will be two in-person clinic visits and 2 phone calls.
Up to $370.00 compensation will be provided if all study requirements are met.
Screening Dates
Contact us for new screening dates!
Screening Location(s)
CIR Outpatient Clinic @ Johns Hopkins East Baltimore Campus
855 N. Wolfe Street Suite 601
Baltimore, MD 21205
Study Period
6 months
Compensation
Up to $370.00
